Acute Oral Toxicity of a Novel Pharmaceutical Excipient (Grewia gum) in Wistar Rats
DOI:
https://doi.org/10.26538/tjdr/v2i6.3Keywords:
Grewia gum, Excipient, Acute toxicity, Oral, RatAbstract
Purpose: Grewia gum is reported to possess significant potential as a natural pharmaceuti cal excipient. However, there is currently a lack of information regarding its toxicity in animal models.
Methods: The acute oral toxicity of Grewia gum (G. mollis) was evaluated in female Wistar rats using a fixed-dose procedure as outlined in Organisation for Economic Co-operation and Development Guideline No. 420. The animals were monitored for mortality, behavioural, biochemical, and haematological changes and their vital organs were preserved for histopathological analysis.
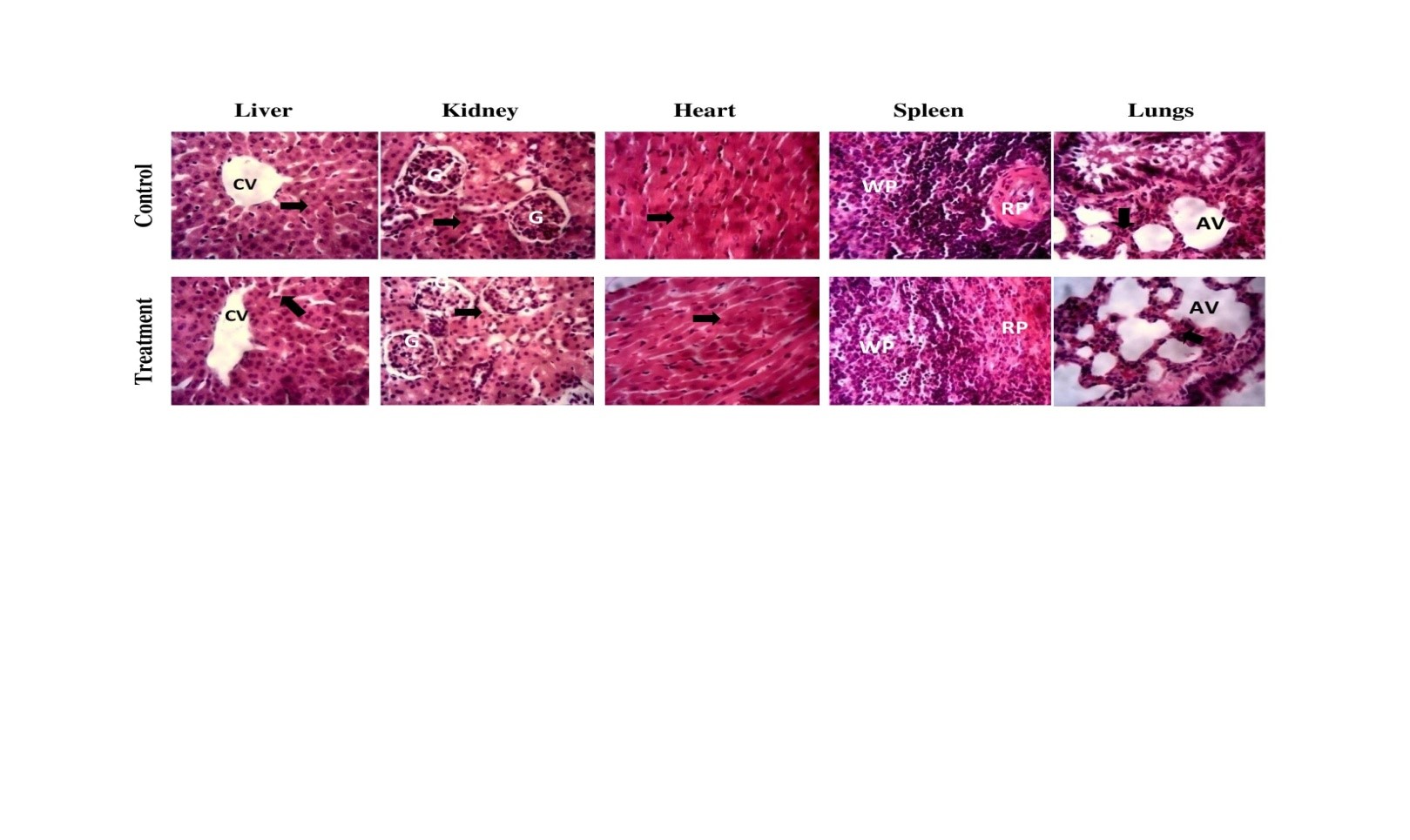
Results:The results indicated no mortality, systemic toxicity, or behavioural changes in the rats administered Grewia gum, with the oral median lethal dose (LD50) exceeding 2000 mg/kg. Grewia gum did not affect food and water consumption in the animals. However, the concentrations of serum bicarbonate, total bilirubin and total protein increased significantly (p < 0.05) in the Grewia gum-treated animals. Furthermore, the administration of Grewia gum resulted in a significant reduction in mean corpuscular haemoglobin concentration (MCHC) values, which suggests subtle effect on haematopoietic tissue. Microscopic examinations of organ sections from both the control and Grewia gum-treated groups revealed no abnormalities.
Conclusion:Thus, oral administration of Grewia gum resulted in mild effects on serum liver and haematological indices, indicating that the gum is safe at the present level of exposure in rats.
Downloads
References
1. Lustig RH. Fructose 2.0: Metabolic, Nep EI, Sims IM, Kontogiorgos V, Morris G, Smith AM. Evaluation of some important physicochemical properties of starch free Grewia gum.Food Hydrocoll. 2016; 53:134-140. https://doi.org/10.1016/j.foodhyd.2015.02.016
2. Nep EI, Odumosu PO, Ngwuluka NC, Olorunfemi PO, Ochekpe NA. Pharmaceutical roperties and applications of a natural polymer from Grewia mollis. J Polym. 2013; Article ID 938726. https://doi.org/10.1155/2013/938726
3. Okafor, I.S., Chukwu, A., and Udeala, K.(2001). Some physicochemical properties of grewia gum. Nigeria.J. Polym. Sci. and Tech. 2001; 2(1):161-167.
4. Nep EI, Conway BR. Characterization of grewia gum, a potential pharmaceutical excipient. J Excip Food Chem. 2010; 1 (1):30-40.
5. Akdowa EP, Boudjeko T, Woguia AL, Gaiani NC, Scher J, Mbofung CMF. Optimization of variables for aqueous extraction of gum from Grewia mollis powder. J Polym. 2014;Article ID 926850. https://doi.org/10.1155/2014/926850
6. Emeje M, Isimi C, Kunle O. Effect of Grewia gum on the mechanical properties of Paracetamol tablet formulations. Afr J Pharm Pharmacol. 2008; 2:1–6
7. Ogaji IJ, Hoag SW. Effect of Grewia gum as a suspending agent on ibuprofen paediatric formulation. AAPS PharmSciTech. 2011; 12 (2): 507- 513. https://doi.org/10.1208/s12249-011-9606-1
8. Muazu J, Musa H, Musa KY. Compression, mechanical and release properties of paracetamol tablets containing acid treated Grewia gum. J Pharm Sci Technol.2009; 1 (2):74-79.
9. Nep EI, Conway BR. Physicochemical characterization of grewia polysaccharide gum: effect of drying method. CarbohydrPolym.2011;84 (1): 446–453. https://doi.org/10.1016/j.carbpol.2010.12.005
10. Nep EI, Conway BR.Grewia gum 2: mucoadhesive properties of compacts and gels. Trop J Pharm Res.2011; 10 (4): 393-401. https://doi.org/10.4314/tjpr.v.10i4.4
11. Nep EI, Conway BR. Polysaccharide gum matrix tablets for oral controlled delivery of cimetidine. J Pharm Sci Res. 2010; 2(11):708-716.
12. Nep EI, Conway BR.Evaluation of Grewia polysaccharide gum as a suspending agent. Int J Pharm Pharm Sci. 2011; 3 (2):168-173.
13. Nep EI, Conway BR.Preformulation studies on Grewia gum as a formulation excipient. J Therm Anal Calorim.2012; 108 (1):197–205. https://doi.org/10.1007/s10973-011-1782-4
14. Ologunagba MO, Kolawale OM, Echerenwa AN, Silva BO. Development and characterization of capsaicin creams formulated with Grewia mucilage-HPMC base. JSciPract Pharm. 2020; 7(1):365-375. http://dx.doi.org/10.47227/jsppharm.v7i1.3
15. National Research Council (2011). Guide for the care and use of laboratory animals. 8th edition. Department of Health and Human Services, National Institutes of Health. National Academies Press,Washington D.C. pp 41-132
16. Organisation for Economic Cooperation and Development (OECDa) (2001). Acute oral toxicity- fixed dose procedure. OECD Guideline 420 for Testing of Chemicals. OECD Publishing, Paris.
17. Zarei L, Shahrooz R. Protective effects of cornus mas fruit extract on methotrexate-induced alterations in mice testicular tissue: evidences for histochemical and histomorphometrical changes in an animal model study. Vet Res Forum. 2019; 10 (4):307-313.
https://doi.org/10.30466/vrf.2019.69516.1955.
18. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18 (6):499–502.
19. Suvarna KS, Layton C, Bancroft JD. Bancroft’s theory and practice of Histological techniques. 8th ed. London; Elsevier Health Sciences; 2018. pp40-183.
20. Ghasemi A, Jeddi S, Kashfi K. The laboratory rat: age and body weight matter. EXCLI J.2021; 20: 1431-1445. https://doi.org/10.17179/excli2021-4072
21. Lai MN, Hsu HC, Ng LT. Safety assessment of the standardised aqueous extract from solid-state cultured Xylarianigripes (Wuling Shen) in rats. ClinPhytosci. 2021; 7(44):1-10. https://doi.org/10.1186/s40816-021-00281-5.
22. Loi B, Fantini N, Colombo G, Gessa GL, Riva A, Bombardelli E, et al.Reducing effect of an extract of Phaseolus vulgaris on food intake in mice-focus on highly palatable foods. Fitoterapia 2013; 85:14-19
23. Ugwah-Oguejiofor CJ, Okoli CO, Ugwah-Oguejiofor M, Umar ML, Ogbulie CS, Mshelia HE, et al.Acute and sub-acute toxicity of aqueous extract of aerial parts of Carrallumadalzielli N.E Brown in mice and rats. Heliyon 2019; 5:e01179. https://doi.org/10.1016/j.heliyon.2019e01179
24. Chiranthanut N, Lertprasertsuke N, Srisook E, Srisook K. Acute and subchronic oral toxicity assessment of extract from Etlingerapavieana rhizomes.Toxicol Rep. 2022; 9:1472-1483. https://doi.org/10.1016/j.toxrep.2022.07.005
25. Ahmadabad MA, Naeimi A, Keymoradzadeh A, Faghani S, Ahmadabad MA, Boroujeni NA, et al. Evaluation of De Ritis (AST/ALT), ALP/ALT, and AST/ALP ratios as prognostic factors in patients with acute ischemic stroke. BMC Neurol. 2022; 22:450. https://doi.org/10.1186/s12883-022-02989-4
26. Levick C. How to interprete liver function tests. South Sudan Med J. 2017; 10(2): 40-43.
27. Creeden JF, Gordon DM, Stec DE, Hinds-Jr TD. Bilirubin as a metabolic hormone: the physiological relevance of low levels. Am J Physiol Endocrinol Metab. 2021; 320: E191- E207. https://doi.org/10.1152/ajpendo.00405.2020
28. Ruiz ARG, Crespo J, Martinez RML, Iruzubieta P, Mercadal GC, Garces ML, et al.Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med.2021; 2(3): 352-361.
https://doi.org/10.1515/almed-2021-0047
29. Asensio M, Ortiz-Rivero S, Morente-Carrasco A, Marin JJG. Etiopathogenesis and pathophysiology of cholestasis. Explor Dig Dis. 2022; 1: 97- 117. https://doi.org/10.37349/edd.2022.00008
30. Baghdasaryan A, Fuchs CD, Österreicher CH, Lemberger UJ, Halilbasic E, Påhlman I, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol.2016;64(3):674-681. https://doi.org/10.1016/j.jhep.2015.10.024.
31. Al-Bassam MM, Al-Saeed HH, Arif HS. Correlation of bilirubin and alkaline phosphatase in infantile patients with cholestasis.Med J Babylon 2019; 16:47-50. http://dx.doi.org/10.4103/MJBL.MJBL_122_18
32. Onofrio FQ, Hirschfield GM. The pathophysiology of cholestasis and its relevance to clinical practice.Clin Liver Dis. 2020; 15 (3): 110-114. https://doi.org/10.1002/cld.894.
33. Levitt MD, Hapak SM, Levitt DG. Alkaline phosphatase pathophysiology with emphasis on the seldom-discussed role of defective elimination in unexplained elevations of serum ALP-A case report and literature review. Clin Exp Gastroenterol.2022; 15:41–49. https://doi.org/10.2147/CEG.S345531.
34. Yu L, Liu Y, Wang S, Zhang Q, Zhao J, Zhang H, et al.Cholestasis: exploring the triangular relationship of gut microbiota-bile acid-cholestasis and the potential probiotic strategies.Gut Microbes 2023; 15:1, 2181930.https://doi.org/10.1080/19490976.2023.2181930.
35. Jeong SM, Choi S, Kim K, Kim SM, Lee G, Park SY, et al.Effect of Change in Total Cholesterol Levels on Cardiovascular Disease Among Young Adults. J Am Heart Assoc.2018; 7(12): 1-17.
https://doi.org/10.1161/JAHA.118.008819.
36. Jung E, Kong SY, Ro YS, Ryu HH, Shin SD. Serum cholesterol levels and risk of cardiovascular death: a systematic review and a dose-response meta-analysis of prospective cohort studies. Int J Environ Res Public Health 2022; 19(14):1-12. https://doi.org/10.3390/ijerph19148272.
37. Kim HJ, Jeong S, Oh YH, Park SJ, Cho Y, Park SM. Changes in high-density lipoprotein cholesterol with risk of cardiovascular disease among initially high-density lipoprotein-highparticipants.CardiovascDiabetol.2023; 22:71. https://doi.org/10.1186/s12933-023-01805-8
38. Martins J, Steyn N, Rossouw HM, Pillay TS. Best practice for LDL-cholesterol : when and how to calculate.J Clin Pathol. 2023; 76: 145- 152. https://doi.org/10.1208/S12249-011-9606-1
39. Sirtori CR, Corsini A, Ruscica M. The role of high-density lipoprotein cholesterol in 2022. Curr Atheroscler Rep. 2022; 24:365-377. https://doi.org/10.1007/s11883-022-01012-y
40. Banach M, Penson PE, Farnier M, Fras Z, Latkovskis G, Laufs U, et al. Pempedoic acid in the management of lipid disorders and cardiovascular risk, 2023 position paper of the International Lipid Expert Panel (ILEP). Prog Cardiovasc Dis. 2023; 79: 2-11. https://doi.org/10.1016/j.pcad.2023.03.001
41. Do C,Vasquez PC, Soleimani M. Metabolic alkalosis pathogenesis, diagnosis, and treatment:core curriculum. Am J Kidney Dis. 2022; 80(4):536-551. 2022. https://doi.org/10.1053/j.ajkd.2021.12.016
42. Misaka T, Sato Y, Sugawara Y, Ogawara R, Ichimura S, Tomita Y, Anzai F, Yokokawa T, Sato A,Shimizu T, Sato T, Oikawa M, Kobayashi A, Yoshihisa A, Takeishi Y. Elevated blood bicarbonate levels and long-term adverse outcomes in patients with chronic heart failure. ESC Heart Fail. 2024; 11:4420-4426
43. Briggs C, Bain BJ (2017). Basic haematological techniques. In: Bain BJ, Bates I, Laffan MA, Lewis SM (Eds). Dacie and Lewis Practical haematology.Twelve edition. Elsevier Limited, pp18- 49.
44. Zhang Z, Gao S, Dong M, Luo J, Xu C, Wen W, et al.Relationship between red blood cell indices (MCV, MCH, and MCHC) and major adverse cardiovascular events in anaemic and nonamemic patients with acute coronary syndrome. Dis. Markers 2022, Article ID 2193343, https://doi.org/10.1155/2022/2193343
45. Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, et al. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. ToxicolPathol.2007; 35(5): 751-755. https://doi.org/10.1080/01926230701595300.
46. Lazic SE, Semenova E, Williams DP. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci Rep.2020; 10:6625. https://doi.org/10.1038/s41598-020-63465-y.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




