Exploring the efficacy of Calotropis procera methanol leaf extract with standard antimalarial drugs in the murine malaria model
DOI:
https://doi.org/10.26538/tjdr/v2i6.2Keywords:
Antimalarial, Calotropis procera, Plasmodium berghei, Chloroquine, Artesunate, PyrimethamineAbstract
Purpose: The emergence and proliferation of drug-resistant Plasmodium falciparum has critically compromised the efficacy of traditional antimalarial treatments significantly challenging global control efforts. There is a growing scientific focus on exploring plant-based therapies as complementary or alternative options for antimalarial treatment. This investigation sought to examine the antimalarial effect of Calotropis procera methanol leaf extract (MLCP) alone and in combination with standard antimalarial drugs in Plasmodium berghei-infected mice.
Methods: Standard protocols were followed for oral acute toxicity evaluation and phytochemical tests of MLCP. Curative and prophylactic effects of MLCP individually and in combination with chloroquine (CQ), artesunate (ART), and pyrimethamine (PYR), were evaluated using recognized experimental techniques in Plasmodium berghei-infected mice.
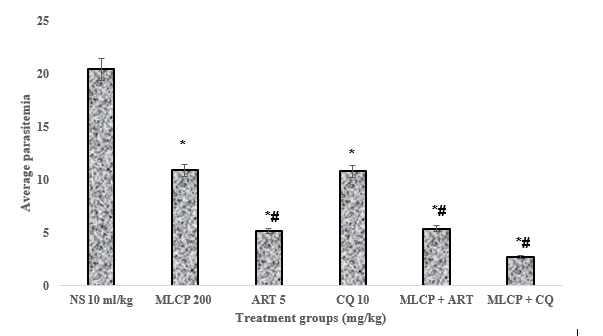
Results: MLCP at 200 and 400 mg/kg doses produced a marked decrease (p < 0.05) in parasitemia levels in both tests. In the curative study, the combination of MLCP + CQ (200/10 mg/kg) and MLCP + ART (200/5 mg/kg) considerably (p < 0.05) reduced parasitemia levels. The percentage of chemosuppression produced by MLCP + CQ (86.9 %) was better than CQ alone (47.2 %). However, the MLCP + ART combination and ART alone produced similar parasite suppressive effects with percentage chemosuppression of 73.6 % and 74.7 %, respectively. In the prophylactic test, the MLCP + PYR (200/1.2 mg/kg) combination produced a chemosuppression of 78.7 % compared to PYR alone which produced a chemosuppression of 78.0 %.
Conclusion: The findings show that combining Calotropis procera leaves with standard antimalarial chloroquine enhanced antimalarial efficacy against Plasmodium berghei infection in mice.
Downloads
References
1. World Health Organization. World Malaria Report 2024. Geneva: World Health Organization; 2024. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024. Accessed 10th March, 2025
2. Plowe CV. Malaria chemoprevention and drug resistance: a review of the literature and policy implications. Malar J. 2022; 21: 104. doi:10.1186/s12936-022-04115-8
3. Habibi P, Shi Y, Fatima Grossi-de-Sa M, Khan I. Plants as sources of natural and recombinant antimalarial agents. Mol Biotechnol. 2022; 64(11): 1177-1197. doi:10.1007/s12033-022-00499-9
4. Efferth T, Kaina B. Toxicity of the antimalarial artemisinin and its derivatives. Crit Rev Toxicol. 2010; 40(5): 405-421. doi:10.3109/10408441003610571
5. Maafoh C, Onyedibe K. Alternative first-line malaria treatment. Ann Afr Med. 2024; 23(1): 5-12. doi:10.4103/aam.aam35_23
6. Erhirhie EO, Ikegbune C, Okeke AI, Onwuzuligbo CC, Madubuogwu NU, Chukwudulue UM, Okonkwo OB. Antimalarial herbal drugs: a review of their interactions with conventional antimalarial drugs. Clin Phytosci. 2021; 7: 4. doi:10.1186/s40816-020-00242-4
7. Ginsburg H, Deharo E. A call for using natural compounds in the development of new antimalarial treatments. Malar J. 2011; 10(Suppl 1): S1. doi:10.1186/1475-2875-10-S1-S1
8. Mossa JS, Tariq M, Mohsin A, Ageel AM, Al-Yahya MA, Al-Said MS, Rafatullah S. Pharmacological studies on aerial parts of Calotropis procera. Am J Chin Med. 1991; 19(3-4): 223-231. doi: 10.1142/S0192415X91000302.
9. Rasik AM, Raghubir R, Gupta A, Shukla A, Dubey MP, Srivastava S, Jain HK, Kulshrestha DK. Healing potential of Calotropis procera on dermal wounds in
Guinea pigs. J Ethnopharmacol. 1999; 15; 68(1-3): 261-266. doi: 10.1016/s0378-8741(99)00118-x
10. Meena AK, Yadav A, Rao MM. Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J Tradit Med. 2011; 6(2): 45-53.
11. Elimam AM, Elmalik KH, Ali FS. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J Biol Sci. 2009;16(2): 95-100. doi: 10.1016/j.sjbs.2009.10.007
12. Shobowale OO, Ogbulie NJ, Itoandon EE, Oresegun MO, Olatope SOA. Phytochemical and antimicrobial evaluation of aqueous and organic extracts of Calotropis procera Ait leaf and latex. Niger Food J. 2013; 31(1): 77-82.
13. Alrheam AIAA, Shehri ZSA. Ethnopharmacological study of the aqueous, chloroform, ethanol leaves extracts and latex of Calotropis procera in diabetic rats. Biomed Res Ther. 2015; 2:1-6.
14. Patil RA, Makwana AB. Anti-hyperbilirubinemic and wound healing activity of aqueous extract of Calotropis procera leaves in Wistar rats. Indian J Pharmacol. 2015; 47(4): 398-402. doi: 10.4103/0253-7613.161262.
15. Abdulkadir A, Umar IA, Ibrahim S, Onyike E, Kabiru AY. Cysteine protease inhibitors from Calotropis procera with antiplasmodial potential in mice. J Adv Med Pharm Sci. 2016; 6(3): 1-13.
16. Kutelu AM, Okwute SK. Phytochemical analysis, volatile components, and biological evaluation of the leaf of Calotropis procera (Asclepiadaceae). Int J Res Innov Appl Sci. 2019; 4(11): 60-67.
17. Ahmad Nejhad A, Alizadeh Behbahani B, Hojjati M, Vasiee A, Mehrnia MA. Identification of phytochemical, antioxidant, anticancer, and antimicrobial potential of Calotropis procera leaf aqueous extract. Sci Rep. 2023; 13(1):14716. doi: 10.1038/s41598-023-42086-1.
18. Murshed M, Al-Tamimi J, Mares MM, Hailan WAQ, Ibrahim KE, Al-Quraishy S. Pharmacological effects of biosynthesis silver nanoparticles utilizing Calotropis procera leaf extracts on Plasmodium berghei-infected liver in experimental mice. Int J Nanomedicine. 2024; 19:13717-13733. doi: 10.2147/IJN.S490119.
19. Muregi FW, Chhabra SC, Njagi EN, Lang’at-Thoruwa CC, Njue WC, Orago AS, Omar SA, Ndiege IO. In vitro antiplasmodial activity of some plants used in Kisii, Kenya, against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 2003; 84: 235-239.
20. Adibe MO. Prevalence of concurrent use of herbal and synthetic medicines among outpatients in a mission hospital in Nigeria. Int J Drug Dev Res. 2009; 1: 60-66.
21. Anagu OL, Attama AA, Okore VC, Gugu HT, Ngene AA, Esimone CO. Azadirachta indica extract-artesunic acid combination produces an increased cure rate of Plasmodium berghei-infected mice. Pharm Biol. 2014; 52(7): 883-889. doi: 10.3109/13880209.2013.872153.
22. Osei SA, Biney RP, Obese E, Agbenyeku MA, Attah IY, Ameyaw EO, Boampong JN. Xylopic acid-amodiaquine and xylopic acid-artesunate combinations are effective in managing malaria in Plasmodium berghei-infected mice. Malar J. 2021; 20:113. doi: 10.1186/s12936-021-03658-6.
23. Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004; 3(6): 509-520. doi: 10.1038/nrd1416
24. National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011.
25. Evans WC. Trease and Evans Pharmacognosy. 16th ed. Elsevier; 2009. p. 10-11.
26. Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54(4): 275-287. doi: 10.1007/bf01234480.
27. Ryley JF, Peters W. The antimalarial activity of some quinoline esters. Ann Trop Med Parasitol. 1970; 84: 209-22.
28. Peters W. Drug resistance in Plasmodium berghei: Chloroquine resistance. Exp Parasitol. 1965;17: 80-87.
29. Iwalewa EO, Lege-Oguntoye L, Rai PP, Iyaniwura TT. In vivo and in vitro antimalarial activity of two crude extracts of Cassia occidentalis leaf. Niger J Pharm Sci. 1997; 5: 23-28.
30. Ukwe CV, Ekwunife OI, Epueke EA, Ubaka CM. Antimalarial activity of Ageratum conyzoides in combination with chloroquine and artesunate. Asian Pac J Trop Med. 2010; 3(12): 943-947.
31. Mohammed A, Ibrahim SI, Bilbis LS. Toxicological investigation of aqueous leaf extract of Calotropis procera (Ait.) R. Br. in Wistar albino rats. Afr J Biochem Res. 2012; 6(7): 90-97.
32. Kazeem MI, Mayaki AM, Ogungbe BF, Ojekale AB. In-vitro studies on Calotropis procera leaf extracts as inhibitors of key enzymes linked to diabetes mellitus. Iran J Pharm Res. 2016; 15(Suppl): 37-44.
33. Kalu AO, Egwim EC, Jigam AA, Muhammed HL. Green synthesis of magnetite nanoparticles using Calotropis procera leaf extract and evaluation of its antimicrobial activity. Nano Express. 2022; 3(4): 045004. doi: 10.1088/2632-959X/aca925
34. Mamat A, Lame Y, Adeline FY, Yvette N, Ndode Herman ON, Arnold Roger BN, Dieudonné N. In vitro nematocidal potential of hydro-ethanolic and aqueous extracts of Calotropis procera (Aiton) WT Aiton, 1811 (Apocynaceae) and Faidherbia albida (Delile) A. Chev., 1934 (Fabaceae) against Onchocerca ochengi and Caenorhabditis elegans. Heliyon. 2023; 9(5): e16379. doi: 10.1016/j.heliyon.2023.e16379.
35. Rabizadeh F, Mirian MS, Doosti R, Kiani-Anbouhi R, Eftekhari E. Phytochemical classification of medicinal plants used in the treatment of kidney disease based on traditional Persian medicine. Evid Based Complement Alternat Med. 2022; 2022:8022599. doi: 10.1155/2022/8022599.
36. Correa Soares JB, Menezes D, Vannier-Santos MA, Ferreira-Pereira A, Almeida GT, Venancio TM, Verjovski-Almeida S, Zishiri VK, Kuter D, Hunter R, Egan TJ, Oliveira MF. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl Trop Dis. 2009; 3(7): e477. doi: 10.1371/journal.pntd.0000477.
37. Zheng D, Liu T, Yu S, Liu Z, Wang J, Wang Y. Antimalarial mechanisms and resistance status of artemisinin and its derivatives. Trop Med Infect Dis. 2024; 9: 223. doi: 10.3390/tropicalmed9090223.
38. Hidayati AR, Widyawaruyanti A, Ilmi H, Tanjung M, Widiandani T, Siswandono DS, Hafid AF. Antimalarial activity of flavonoid compound isolated from leaves of Artocarpus altilis. Pharmacogn J. 2020; 12(4): 835-842.
39. Nwonuma CO, Balogun EA, Gyebi GA. Evaluation of antimalarial activity of ethanolic extract of Annona muricata L.: An in vivo and in silico approach. J Evid Based Integr Med. 2023; 28. doi: 10.1177/2515690X231165104.
40. Sharma S, Ali M, Hussain A. Pharmacological activities of Calotropis procera extracts: A review. Asian Pac J Trop Biomed. 2013; 3(8): 641-646.
41. Kinda PT, Guenné S, Compaoré M, Balé B, Alin C, Raymond B, Martin K. Toxicological characterization and central nervous system effects of Calotropis procera Ait. aqueous extracts in mice. Asian Pac J Trop Med. 2019;12(7): 329-336. doi: 10.4103/1995-7645.262077
42. Eluwa MA, Idumesaro NE, Ekong MB, Akpantah AO. Comparative toxicity study of methanolic extract of Calotropis procera leaves in rats. Afr J Tradit Complement Altern Med. 2016; 13(1): 234-243.
43. Clark J, Ortego LS, Fairbrother A. Sources of variability in plant toxicity testing. Chemosphere. 2004; 57(11): 1599-1612. doi: 10.1016/j.chemosphere.2004.07.044.
44. Adugna M, Feyera T, Taddese W, Admasu P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Glob J Pharmacol. 2014; 8(3): 460-468.
45. Peter IT, Anatoli VK. The current global malaria situation. Malaria parasite biology, pathogenesis and protection. Washington DC: ASM Press; 1998. p. 11-22.
46. Egan TJ. Haemozoin formation. Mol Biochem Parasitol. 2008; 157(2): 127-136. doi: 10.1016/j.molbiopara.2007.11.005.
47. Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003; 424(6951): 957-961. doi: 10.1038/nature01813.
48. Iwalokun BA. Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine-resistant and sensitive Plasmodium berghei strains. Afr Health Sci. 2008; 8(1): 25-35.
49. Sibhat GG, Hiben MG. Consequence of concurrent use of chloroquine and hydroalcoholic extract of Balanites aegyptiaca and leaf latex of Aloe camperi. J Phytopharmacol. 2016; 5(1): 35-37.
50. Voravuth S, Awatsada D, Pinanong O. Antimalarial activity of kaempferol and its combination with chloroquine in Plasmodium berghei infection in mice. J Pathog. 2018; 2018: 3912090. doi: 10.1155/2018/3912090.
51. Onaku LO, Attama AA, Okore VC, Tijani AY, Ngene AA, Esimone CO. Antagonistic antimalarial properties of pawpaw leaf aqueous extract in combination with artesunic acid in Plasmodium berghei-infected mice. J Vector Borne Dis. 2011; 48(2): 96-100.
52. Ocloo A, Okpattah WE, Quasie O, Sakyiamah MM, Okine LKN. Concurrent administration of aqueous extract of Cryptolepis sanguinolenta reduces the effectiveness of artesunate against Plasmodium berghei in rats. J Appl Pharm Sci. 2014; 4(3): 024-028. doi: 10.7324/JAPS.2014.40306
53. Ihekwereme CP, Agafuchukwu P, Erhirhie EO. Artesunate antagonizes antiplasmodial activity of Vernonia amygdalina methanol leaf extract in murine model of malaria. Am J Physiol. 2020; 10(1): 1-6. doi: 10.5455/ajpbp.20190822021151
54. Somsak V, Borkaew P, Klubsri C, Dondee K, Bootprom P, Saiphet B. Antimalarial properties of aqueous crude extracts of Gynostemma pentaphyllum and Moringa oleifera leaves in combination with artesunate in Plasmodium berghei-infected mice. J Trop Med. 2016; 2016: 8031392. doi: 10.1155/2016/8031392.
55. Idowu OA, Babalola AS, Olukunle J. Antagonistic effects of some commonly used herbs on the efficacy of artemisinin derivatives in the treatment of malaria in experimental mice. Bull Natl Res Cent. 2020; 44: 1-8. doi.org/10.1186/s42269-020-00429-2
56. Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006; 36: 1427-1441.
57. Hansen DS. Inflammatory responses associated with the induction of cerebral malaria: Lessons from experimental murine models. PLoS Pathog. 2012; 8: e1003045. doi: 10.1371/journal.ppat.1003045
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




